Scandals involving breast implants and all-metal hips prosthetics have raised global concerns and prompted regulatory reform in the European Union. Now the International Consortium of Investigative Journalists (ICIJ) has conducted a major investigation into the medical device sector.
The debacle that is the DePuy metal hip illuminates the ways medical devices are brought to market and the mayhem that can ensue if a product fails to live up to expectations.
On a per capita basis, more of the DePuy hips were implanted in Ireland than in any other jurisdiction. In excess of 1,000 Irish patients have issued proceedings against DePuy. Hundreds have already settled.

Gillian O’Sullivan’s story
When Gillian O'Sullivan gets up from her chair to fetch some hip X-rays, you can't but notice that one of her legs is shorter than the other.
The 54-year-old mother-of-three from Tramore, Co Waterford, was born with a congenital deformity of the hips and spent a year and two months in hospital in Waterford as a child. During the 1980s she attended the orthopaedic surgeon, and founder of the Blackrock Clinic, Jimmy Sheehan. "He told me that I would need hips [implants] later on."
One of the difficulties with replacing hips is that the artificial implants wear out. As bone and tissue get destroyed when prosthetics are being implanted, replacing worn-out implants with new ones is not a simple matter. For this reason, the practice has been to wait until people are older, and less active, before giving them artificial hips. If you get the timing right, the patient may be dead before the hip implant has failed.
But in the early 2000s, hip implant manufacturers started to market new, longer-lasting implants they said were suited to younger, active people.
In 2005, when she was 40, Gillian O'Sullivan went to see the highly regarded local orthopaedic surgeon, Tadhg O'Sullivan O'Sullivan, in the Waterford Regional Hospital. She was suffering from arthritis.
“He told me there was a lovely new hip on the market that could last a lifetime . . . I would never look back.”
Her surgeon was referring to a new metal product from DePuy, a subsidiary of Johnson & Johnson and a major manufacturer of orthopaedic products. The so-called ASR hip came in two variants. One was a hip resurfacing device, which covered the cup and ball of the hip socket, while the second was a total hip replacement product, which involved replacing the top of the femur with a metal device, which then sat into the metal cup.
Both products differed from what was previously on the market in that both traditional hip implants used a metal ball and a polyethylene or plastic cup. The DePuy hip had a ball and cup made out of a hard-wearing cobalt chromium metal alloy.
Tadhg O’Sullivan was one of six design surgeons who had worked with DePuy’s engineers to design the new product. It is standard practice for innovative new products to be developed with the assistance of clinicians.
At the time he saw Gillian O’Sullivan, the Waterford surgeon was showing surgeons around the world how to implant the new product, and taking part in post-market surveillance of how the product was performing.

In October 2005, Gillian O’Sullivan had a DePuy hip replacement device implanted on her left side at the Kilcreene Orthopaedic Hospital in Kilkenny. In May 2006, her second hip was replaced.
She was told she now had the “hips of Brian O’Driscoll”.
However, her operations were difficult and she experienced pain afterwards.
“By Christmas 2006 we knew I was in trouble. I had no energy levels, I was really sick, and you could hear me coming down the stairs. There was a click, click, click, every time I moved.”
Her children could hear the grinding of her metal hips.
In May 2007, she went back to Tadhg O’Sullivan. “I said, I’m in trouble here. He argued with me. He said I was in better condition than I had been.”
She was told that “the god of orthopaedics” had performed her hip operation and that she should wait and see.
By this stage O'Sullivan had moved from the Waterford Regional Hospital to the private Whitfield Clinic, also in Waterford.
“Between 2007 and 2010, I was in an awful state. I would say to myself, I think I am dying.”
You need to get those hips out now. They're poisoning you
Gillian O’Sullivan now believes that during the years 2007 to 2011, metal ions from her hip implants were progressively damaging the flesh and bone of her hip joints.
When the DePuy hip was withdrawn from the market in August 2010, Irish patients were advised that their future care would involve the monitoring of the cobalt and chromium levels in their blood.
After the withdrawal, Gillian O’Sullivan got a letter for an appointment at the Whitfield and Tadhg O’Sullivan warned her that she would be hearing a lot of public debate about cobalt and chromium ion levels in the blood. He said it was “all bullshit”.
“They are trying to frighten everybody.”
She went to see Jimmy Sheehan at the Blackrock Clinic again and brought along her blood test results which showed very high metal ion levels. "He said, you need to get those hips out now. They're poisoning you."
In 2011, the right hip was replaced by O’Sullivan at the Whitfield, and a year later the second hip was also replaced.
The operations, which were paid for by DePuy, were difficult, she said, and left her with a left leg that was longer than her right.
Kiera Kenneally’s story
During her original operations and subsequent operations Gillian O'Sullivan met a number of other patients who were having DePuy hips implanted or replaced by Tadhg O'Sullivan. Among those she met was Kiera Kenneally, who had DePuy hips implanted by Tadhg O'Sullivan in 2005 and 2006, the first when she was just 16 years old.
Kenneally, who now lives in Sandyford, Dublin, is originally from Hillview, Waterford, and Gillian O'Sullivan knew her family. Kenneally sued DePuy. Her case was heard over a number of days in the High Court in 2016, but the case was settled with the agreement including a confidentiality clause. However, the transcripts from the sitting days have been seen by The Irish Times.
In August 2005, Kenneally had a resurfacing procedure done by O’Sullivan in Kilcreene. In May of the following year, the second hip was resurfaced.
The left hip implant was replaced by O’Sullivan in 2010. During the operation, substantial metal ion damage to her hip tissue was discovered and there was substantial loss of bone.
Blood tests taken afterwards showed cobalt and chromium blood levels that were, respectively, 10 and four times above what would be expected, the High Court was told.
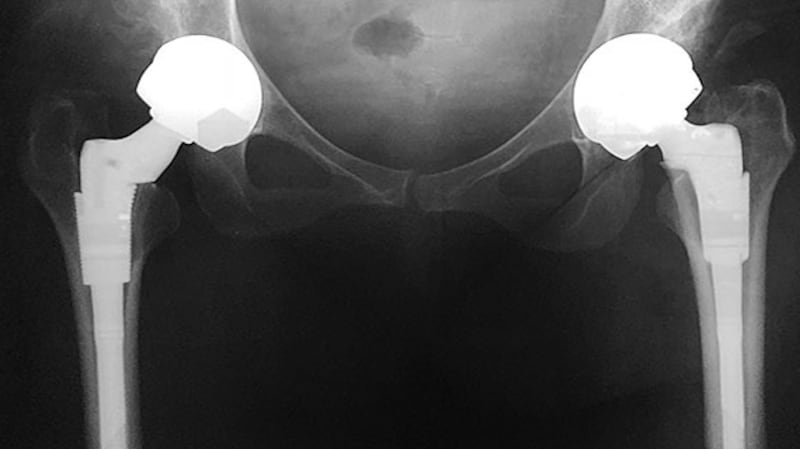
Kenneally has since been advised she may in time need to have a Girdlestone procedure, which fuses the femur to the hip so that it can no longer bend. She may end up in a wheelchair.
During her evidence, Kenneally told the court about coming across the word Girdlestone in a report drawn up about her case by the English surgeon Tony Nargol. "I said 'Where's my Google because I don't know what that means.' I Googled it and obviously it was terrifying . . . I do remember reading what it said on Google and passing the phone to [her husband] Declan and then crying."
The medical records in Kenneally’s case showed that before her procedure, Tadgh O’Sullivan discussed both with her and her mother the concept of metal-on-metal hips, the possibility of failure, and the issue of debris (which in turn leads to the release of ions).
An honest historian
Gillian O’Sullivan also sued DePuy. During her High Court action, she says, DePuy “fought me all the way”.
“They were horrific.”
She also enlisted Nargol as an expert witness and he told the court that 90-95 per cent of her problems were due to the DePuy implants. DePuy argued that 90-95 per cent of her difficulties were due to her congenital condition.
Mr Justice Kevin Cross decided that 90 per cent of O’Sullivan’s difficulties were referable to the DePuy hips, and awarded her €740,000. DePuy said it would appeal and the matter was then settled for €520,000.
Unusually for people who made settlements with DePuy, Gillian O’Sullivan did not agree to a “gagging” clause and so is free to discuss her experience.
In his judgment in November 2016, Mr Justice Cross said O’Sullivan was an “honest historian” who did not consciously exaggerate, but that her difficulties prior to her hip operations were greater than she recalled.
I live my life. If I'm upright for the day, I'm happy.
As was the case with the Kenneally trial, Tadgh O’Sullivan was not a party in the O’Sullivan case, was not called by either side, and no one criticised his surgical skills. The judge said he accepted that O’Sullivan’s relationship with her surgeon had broken down some time after the second DePuy implant operation.
O’Sullivan would need future revisions and would continue to be severely disabled for the rest of her life, the judge said.
“The plaintiff will also be in significant pain, probably for life.”
Gillian O’Sullivan says, reflecting on what’s happened to her: “You make the most of your life. I can’t be on a beach. The balance is completely gone, and I would fall over nothing. I get pains and contractions. I was in bed for six weeks after Christmas.”
She is a mix of being surprisingly upbeat, while also occasionally displaying a fierce sense of resentment towards Tadhg O’Sullivan.
“I live my life. If I’m upright for the day, I’m happy.”
The DePuy hip debacle may end up costing DePuy up to $4 billion (€3.53 billion) in damages. Other producers of metal hip implants are also being sued.
Although figures are not available for the Republic, total damages of up to €80 million may yet be paid to Irish recipients of DePuy hips, with legal costs in addition to that. It is the largest corporate personal injuries episode to ever hit the Irish legal system.
Regulation process
Despite the challenges a metal-on-metal product presented, a new all-metal hip resurfacing product, known as the Birmingham Hip Resurfacing (BHR) system, made by Smith & Nephew, came on the market in the late 1990s and began to prove popular.
The device was attractive for young, active people with hip problems, and Tadhg O’Sullivan was an early fan. A former GAA player himself, O’Sullivan was known for treating young athletes with joint problems
O'Sullivan told The Irish Times that it was because of his extensive use of the Birmingham hip that he was invited to work with DePuy in relation to its new product. At the time "I was probably one of the most experienced hip resurfacing surgeons in the world."
Companies developing new implants often engage surgeons to assist them. Although DePuy engineers put a huge amount of work into designing their new product, this did not including clinical trials, something that was not necessary under EU rules.
Simulator tests were used, but critics say the tests did not replicate the repeated stop and start motion that is intrinsic to human joint use, and were restricted to larger model components, the sort used by larger framed people.
“It would have been a very good design process if they were designing a washing machine,” says Trinity College Dublin engineering professor David Taylor. “They seem to have forgotten that it was going into a human being.”

Taylor, who specialises in materials that are inserted into the body, has acted for a number of people who are suing DePuy. For him, a critical failure made by the DePuy engineers was not testing the proposed product on people prior to going to market.
The head of medical device regulation in Ireland, Dr Niall MacAleenan, speaking generally, said clinical testing of medical device products was complicated by the fact that many devices were designed to be inside the body for years if not decades.
Assessments as to the suitability of implanted devices involves a “risk benefit” assessment. “There is always risk. The question is if it is an acceptable level of risk.”
In January 2004, DePuy got approval from a UK notified body, BSI, to market its two metal hip products throughout the EU. Approval across other, non-EU markets soon followed. For the US market, the company had to get approval from the Food and Drug Administration (FDA), which was considered a tougher regulatory authority than the multi-agency structure operating in the EU.
‘Opinion leader’
English surgeon Tony Nargol was an early enthusiast for the new DePuy product. In September 2003, he travelled to Rome for the product launch, where he met Tadhg O’Sullivan and some of the other design surgeons. He agreed to become involved in a clinical study of the device to be used to seek approval for the product in the United States, and also to become an “opinion leader” for the device in the UK.
Between 2004 and 2009, the Newcastle surgeon implanted about 500 of the metal devices, making him one of the highest users of the product in the world.
However, by late 2006 a number of his patients were presenting with difficulties. Nargol began to study the performance of the DePuy product in his patients, working with hospital colleague Simon Jameson.
Among the matters they began to examine was the role played by the “angle” of implantation of the cup. They also began to notice that it was smaller models of the device, used in smaller framed persons, that appeared to be causing the most difficulties.
When he revised the DePuy hip in some of his patients, Nargol noted a “greenish grey” fluid in the hip joint while doing so. This was “tissue necrosis”, he told the courts in Australia in an affidavit in 2013.
In early 2007, he asked Ian Newell, DePuy’s regional manager in the Newcastle area, if he knew of other cases involving greenish grey fluid and tissue necrosis. “Mr Newell replied that he was ‘not aware of any’,” Nargol said in his affidavit.
The response he got from DePuy caused Nargol to think that the problems he was encountering were caused by his work rather than the product.
At a meeting with Newell and others in a hotel in Durham in September 2007, Nargol discussed the apparent link between smaller devices and high ion levels.
In response, Newell showed him the data from a DePuy study of 89 patients who had received the hips in the UK, Australia, South Africa and Germany. The study subjected them to regular blood-testing to see if their cobalt and chromium ion levels became elevated.
In November 2007, two papers about the DePuy hips were presented at the American Academy of Hip and Knee Surgeons conference in Dallas, Texas, one by Nargol and one by Graham Isaac, the DePuy engineer who led the team that designed the new product. Both flagged problems with the device.
Ion levels
Isaac's paper was entitled Whole Blood Metal Ion Levels after Surface Replacement of the Hip; the effect of Component Positioning, and the authors included Isaac, Tadgh O'Sullivan and the other design surgeons, as well as a surgeon from South Africa. The authors reported that 24 months after their procedures, the ion levels for 50 patients (40 male and 10 female) showed three female (30 per cent) and three males (7.5 per cent) had significantly higher ion levels than would be expected.

These elevated levels were associated with their devices being implanted at particular angles, the conference was told.
DePuy, O’Sullivan and the other authors were saying the negative outcomes were related to how the devices were implanted rather than attributable to any defects in the device.
During 2008, Nargol continued to have regular contact with Isaac, Newell and other DePuy representatives. He also began to notice patients with difficulties whose implants had been put in at an angle that was at or near the “safe zone” that he and his colleagues had identified from their research.
In time, Nargol came to believe that the product was at fault, not his work as a surgeon. The implants put in at particular angles tended to fail earlier than others, and so created the early impression that the angle was the source of the problem.
In August 2008, Nargol stopped using the complete hip replacement product and in early 2009 he stopped using the DePuy resurfacing product also.
A colleague, surgeon David Langton, had by this time noticed a new problem with the DePuy implant called “edge-loading”, which contributed to high wear and debris and was, in Langton’s view, the result of the design of the DePuy product.
During a meeting with Isaac on March 11th, 2009, at DePuy’s offices in Leeds, Langton and Nargol outlined their latest findings.
Nargol said there were problems with the DePuy product even when it was implanted at the recommended angle. There was a “design flaw”, especially with smaller cups.
One of the DePuy representatives at the meeting said, “we’re f**ked”, Nargol said in his Australian affidavit. Isaac said the problem was “multifactorial”, with the factors involved including patient weight and activity levels.
Requests submitted through DePuy for interviews with Isaac and Newell were declined.
Whistleblowers
In time Nargol became one of the harshest critics globally of the DePuy product. He and Langton became involved in litigation around the world as patients sued DePuy for damages. Nargol, along with Langton, gave evidence in a lot of these cases and the surgeons are party to continuing proceedings in the US where, as designated whistleblowers, they stand to make significant amounts of money if DePuy ends up having to pay out to the US Government for implants sold to public health programmes.
Nargol gave evidence on behalf of Gillian O’Sullivan in her case. DePuy attacked Nargol’s credibility on the basis of his “general hostility” to DePuy, his involvement in cases in Ireland and the UK, and Nargol’s “opinion that [DePuy] has acted mala fides [in bad faith]”.
The judge did not have to rule on the mala fides issue because of the position adopted by DePuy in the trial, but he rejected the attack on Nargol's evidence. He said the expert witnesses for both sides had given honest evidence. Nargol would not be interviewed by The Irish Times because of the ongoing litigation in various jurisdictions.
In 2010, by way of his involvement in litigation, Nargol discovered that other surgeons had expressed concerns during the mid-2000s about the DePuy product, contrary to what he said he had been told by DePuy.
All in all, this ASR problem has to date been the worst problem I have faced in my surgical career. It has been a real nightmare
The highly regarded Belfast orthopaedic surgeon, David Beverland, told Nargol he had stopped using the DePuy product in early 2007. Requests from The Irish Times to Beverland for an interview met with no response.
Nargol contacted Beverland in 2010 after being told by a former DePuy employee that the Belfast surgeon had experienced problems with his patients.
In his Australian affidavit, Nargol quoted a 2010 email from Beverland to Isaac: “All in all, this ASR problem has to date been the worst problem I have faced in my surgical career. It has been a real nightmare.”
Nargol also said that in 2010 he was shown emails from the Dutch surgeon Paul Born to a DePuy executive, dated June 2006, in which he said he’d experienced a significant failure rate with DePuy and would no longer be using the product.
Nargol said that on several occasions during 2007 and 2008 he had asked Newell, Isaac and their colleague Magnus Flett if other surgeons were noticing similar problems to him.
“Mr Newell, Mr Flett, and Mr Isaac told me on several occasions that ‘no one else has had any problems with the ASR’.”
The DePuy metal hip was voluntarily recalled in August 2010 after figures from the National Joint Registry for England, Wales and Northern Ireland showed it was performing badly when compared with its peers.
The registry collects information on all joint replacement operations so as to measure the performance of the different implants that are on the market.
In its most recent annual report, the registry said that for the 992,090 hip replacements monitored since it began collecting data in 2002, some 7.27 per cent have been replaced (or revised, as the medical term goes).
The replacement rate would be lower if it were not for metal-on-metal implants which showed a replacement rate of 19-22 per cent. The most successful type of hip replacement, metal-on-polyethylene, had a replacement rate of 4.87 per cent.
“Metal-on-metal resurfacings have a revision rate at 14 years of 14.76 per cent and their use, as with all metal-on-metal bearings, is now very limited.”
Approval process
After DePuy withdrew its product from the market, there was extensive discussion as to how it had been approved for sale in the first place.
The EU and the United States are the world’s two most important bodies when it comes to the regulation of the medical devices sector. In the EU, there are multiple “notified bodies” each of which can award CE marks to proposed new medical devices, thereby allowing them be marketed across the union.
In Ireland, the National Standards Authority of Ireland (NSAI) is the body that licenses new medical devices, while the Health Products Regulatory Authority (HPRA) has a role in monitoring the safety of devices that are on the market.
The DePuy products were approved by BSI, a UK-notified body. Clinical trials were not required under EU rules, even though the products are recognised as among the higher-risk category of medical devices. Approval by an EU-certified body opened the way to the devices being marketed in other economies, including Australia and South Africa. Many of the world’s poorer nations rely on the developed world for the regulation of medical devices.
In the US, the FDA decided that the DePuy hip resurfacing product was a significantly new product and required clinical testing. It allowed the marketing of the total hip replacement product.
In 2009, DePuy was told confidentially by the FDA that it would not be granting approval for the hip resurfacing product after reviewing company studies that showed it had failed prematurely in “significant” numbers. In response the multinational quietly withdrew its request for approval.
The DePuy hip replacement product was approved for the US market by way of a controversial pathway called “substantial equivalence”.
Medical devices can be approved for the market in both the EU and the US if they can be shown to be substantially equivalent to a product already on the market, or if they can be shown to be made of parts that are substantially the same as parts already on the market.
The rule applies even if the device or parts already approved have since been withdrawn, even for poor performance reasons.
As a study in the New England Journal of Medicine put it in January 2013, approval by the FDA by way of substantial equivalence "does not mean that a new device is safe and effective: it means that the device is deemed no less safe and no less effective than a predicate".
The study looked at how the DePuy hip replacement device came to be approved for implantation in US citizens. It found that the lineage of the device involved 95 different devices or device parts that had operated as predicate devices or parts to which DePuy said its new product was substantially equivalent.
The predicate devices included ones that were “discontinued long ago because their risk of revision was so much higher than that of other hip” products, the authors noted.
The NSAI was not able to tell The Irish Times how many higher risk devices it had approved over the past decade by way of equivalence, or how many had been approved after clinical trials, even though the information was sought as far back as September 3rd last.
However, MacAleenan of the HPRA points out that precisely because implanted medical devices designed to last a long time inside the body are hard to subject to pre-market clinical testing, the data that comes from devices that are already on the market is particularly valuable.
There is another interesting aspect to the substantial equivalence phenomenon both here and in the US, according to a Newcastle University biomedical engineer, Tom Joyce, who has extensively studied the DePuy debacle
“It happens all the time that a product comes onto market via substantial equivalence, meaning that it is supposed to be similar to a product already on the market, but it is then marketed as if it is wildly different,” said Joyce, who has worked with Nargol and Langon on behalf of Irish plaintiffs.
The EU is currently introducing a new regime for the certification of medical devices that is in part a response to the DePuy hip and other high-profile failures.
The surgeon’s view
Tadhg O'Sullivan told The Irish Times that up to the date that the DePuy products were withdrawn, he never had a problem with any of his resurfacing patients.
Despite the global controversy over the performance of DePuy hip, there is no mistaking O’Sullivan’s continuing enthusiasm for the product.
During a lengthy interview in his consultation rooms in the Whitfield Clinic, O’Sullivan said the DePuy implant was an important innovation for patients with serious hip problems.
Asked if in his view there was a design flaw with the DePuy hip resurfacing device, his answer was simple: “Not really. If put in properly, no.”
Likewise, he said he did not believe there was a flaw with the ball and cup of the total hip replacement product, though there was a problem with the stem that attached it to the patient’s femur.
He believed that DePuy, and J&J, were probably acting on the advice of lawyers when they decided to withdraw the product from the market. The decision, he says, was the equivalent of saying: “Sue the f**k out of us.”
And that, he said, is what has happened.
[It is] very hard to get at the truth when there is a huge amount of money to be made from suing a company
DePuy has “pumped an outrageous amount of money into putting this thing right. Unfortunately, the more they pump in, the more our legal profession want. They’re like an insatiable dog.”
As well as assisting the DePuy engineers and attending design team meetings over a number of years, O’Sullivan was also the co-inventor of two patents registered in the name of DePuy that were associated with the hip product.
Publicly available information in the US indicates that the two US design surgeons who worked with O’Sullivan on the DePuy project, Tom Schmalzried and Thomas Veil, received a number of million dollars each in royalties and other payments from the manufacturer.
However, O’Sullivan says his royalty income from DePuy was “relatively small” and that if the time he had spent as a design surgeon travelling to meetings and conferences for DePuy had instead been spent working at the Whitfield Clinic he would have been equally well rewarded.
He has earned more from DePuy as a result of the programme it has put in place for patients post the withdrawal of the hip product than he did while working as a design surgeon, he said.
The enormous global medical legal battle that has erupted since the product was recalled has led to a great amount of misinformation coming into the public domain, the surgeon said. “We have poor people coming in here thinking they are going to die.” It is “very hard to get at the truth when there is a huge amount of money to be made from suing a company”. he added.
O’Sullivan said he had not seen one paper which, read closely, showed heightened cobalt and chromium levels causing medical problems. Yet people were coming to him now, he said, with normal ageing problems such as becoming forgetful, and blaming it on their ion levels.
When patients with a metal implant and raised ion levels come to him, what does he advise? “I say you have two options. [Leave it in or take it out.] They say, are [my raised ion levels] doing anything to me? I say, I have no idea.”
The patient may feel fantastic, and may have no problem with their hips, but he tells them the only way to get their ion levels down is “to take out the generator”. It is their choice.
Surgeons want to do what is best for their patients, he said. The DePuy project was about “trying to help people of 30 years of age who needed a hip and not be sending them home and have them not working for the next 20 years.”
As matters now stand, most metal hip products have been withdrawn from the market and all major manufacturers are producing a similar ceramic ball and cup product. “They’re all giving you the basic Ford Focus.”
You try to do something like this, which was totally fantastic, and it costs you $3 billion. Why would you do it?
The threat of being sued means doctors feel pressurised into doing what is safest in terms of legal exposure, rather than what is most appropriate for their patients.
Surgeons know that if they put in a product that has been around for decades, but which fails, they cannot be sued, he said. And manufacturers, if they are going to fear being sued, are deterred from creating new devices.
“Can you imagine [DePuy’s] interest now in bringing out the next new great discovery in hips, because that’s what this was. They have no interest . . . You try to do something like this, which was totally fantastic, and it costs you $3 billion. Why would you do it?”
The loser in the end, he says, because of the stifling of innovation, is the patient.
Irish legal process
A special alternative dispute resolution (ADR) process has been put in place by the Irish courts such is the level of claims that have been lodged against DePuy over its metal hip product.
"The [ADR]process does not involve DePuy admitting liability and there is always a gagging order," Orla Kelly, a solicitor with Cantillons solicitors, Cork, one of the Irish firms representing clients suing DePuy, told The Irish Times.
The highest award to date has been €750,000, but the average is in the region of €100,000. If a settlement is not agreed, a claimant can take their case to court.
As of July, 540 of the 1,108 sets of proceedings lodged against DePuy had been resolved. Many of the proceedings lodged do not qualify for the ADR.
A spokeswoman for DePuy said she would not give a figure for total awards made in Ireland to date.
"DePuy has no greater responsibility than to the patients who use our products," she said in a statement to The Irish Times. "We are committed to the wellbeing of our patients and believe the full history of the ASR system shows we have worked properly and responsibly to improve patients' lives through innovation.
“We will continue to stand by our patients.”



















